library(tidyverse)
library(here) # for easier management of file paths
library(rstan)
rstan_options(auto_write = TRUE) # save compiled Stan object
library(brms) # simplify fitting Stan GLM models
library(shinystan) # graphical exploration
library(posterior) # for summarizing draws
library(bayesplot) # for plotting
library(modelsummary) # table for brms
theme_set(theme_classic() +
theme(panel.grid.major.y = element_line(color = "grey92")))
GLM (generalized linear model) is a general class of statistical models for predicting an outcome variable, Y. It accommodates Y in different types of measurement, such as continuous, counts, ordinal, or categorical. GLM is a generalization of the usual regression model that assumes linear associations and normally distributed errors.
What is Regression?
Regression is a class of statistical techniques to understand the relationship between an outcome variable (also called a criterion/response/dependent variable) and one or more predictor variables (also called explanatory/independent variables). For example, if we have the following scatter plot between two variables (Y and X):
set.seed(1)
x <- round(runif(10, 1, 5), 3)
y <- 0.7 + 0.5 * log(x - 1) + rnorm(10, sd = 0.2)
df <- data.frame(x, y)
ggplot(df, aes(x, y)) +
geom_point() +
# stat_function(fun = function(x) 0.7 + 0.5 * log(x - 1), n = 501) +
xlim(1, 5) +
ylim(-1, 2)
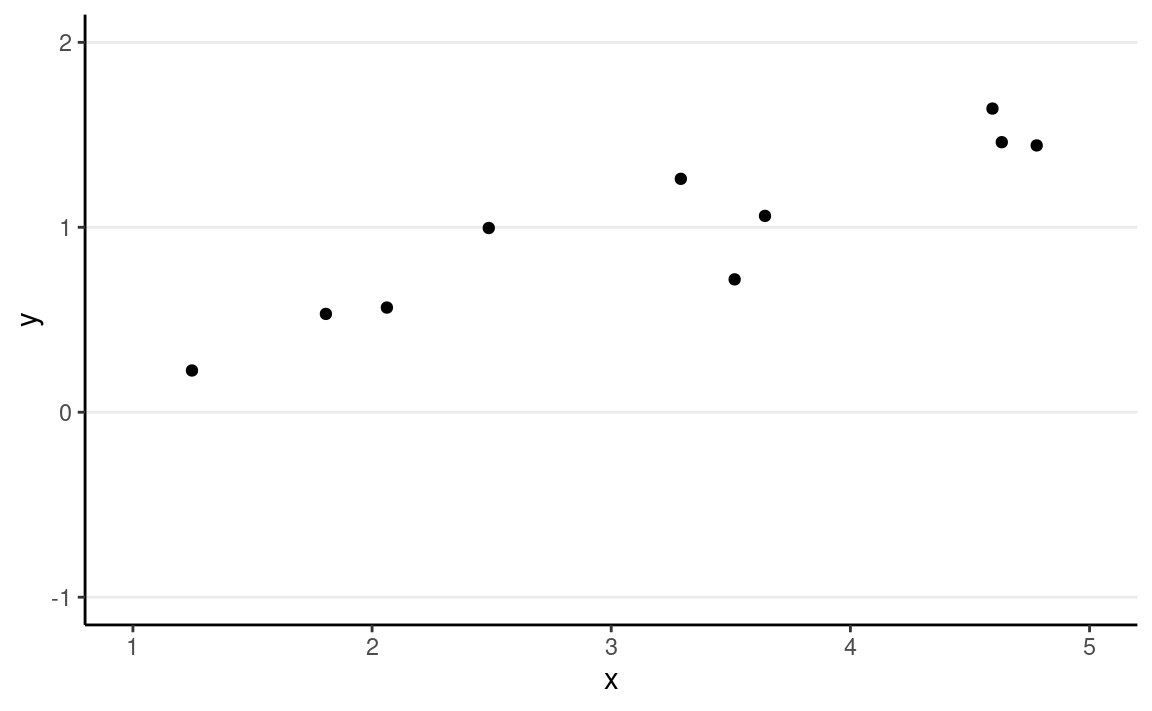
We want to find some pattern from this relationship. In conventional regression, we model the conditional distribution of Y given X, P(Y∣X), by separating the outcome variable Y into (a) a systematic component that depends on the predictor, and (b) a random/probabilistic component that does not depend on the predictor. For example, we can start with a systematic component which only depends on the predictor:

As you can see, all the red dots fall exactly on the curve in the graph above, meaning that as long as one knows the X value, one can predict the Y value with 100% accuracy. We can thus write Y∗=f(X) (where Y∗ is the systematic component of Y).
However, in almost all scientific inquiries, one can never predict with 100% certainty (e.g., there are measurement errors and college randomness in physics). The uncertainty stems from the fact that we rarely measure all the factors that determine Y, and there are genuinely random things (as in quantum physics). Therefore, we need to expand our model to incorporate this randomness, by adding a probabilistic component. Therefore, instead of saying that Y depends just on X, we say Y is random, but the information about X provides information about how Y is distributed. In regression, one studies the conditional distribution P(Y∣X) such that the conditional expectation, E(Y∣X), is determined by X; on top of the conditional expectation, we assume that the observed Y values scatter around the conditional expectations, like the graph on the left below:
p1 <- ggplot(df, aes(x, yhat)) +
stat_function(fun = function(x) 0.7 + 0.5 * log(x - 1), n = 501) +
geom_point(col = "red") +
xlim(1, 5) +
ylim(-1, 2) +
ylab("y") +
geom_curve(aes(x = x, y = yhat + 0.5, xend = x, yend = yhat - 0.5),
curvature = -0.4, col = "red", linetype = "dotdash"
) +
geom_vline(aes(xintercept = x), linetype = "dotted") +
geom_point(aes(x, y), size = 2)
p2 <- ggplot(df, aes(x, y)) +
geom_point(size = 2) +
xlim(1, 5) +
ylim(-1, 2)
gridExtra::grid.arrange(p1, p2, nrow = 1)
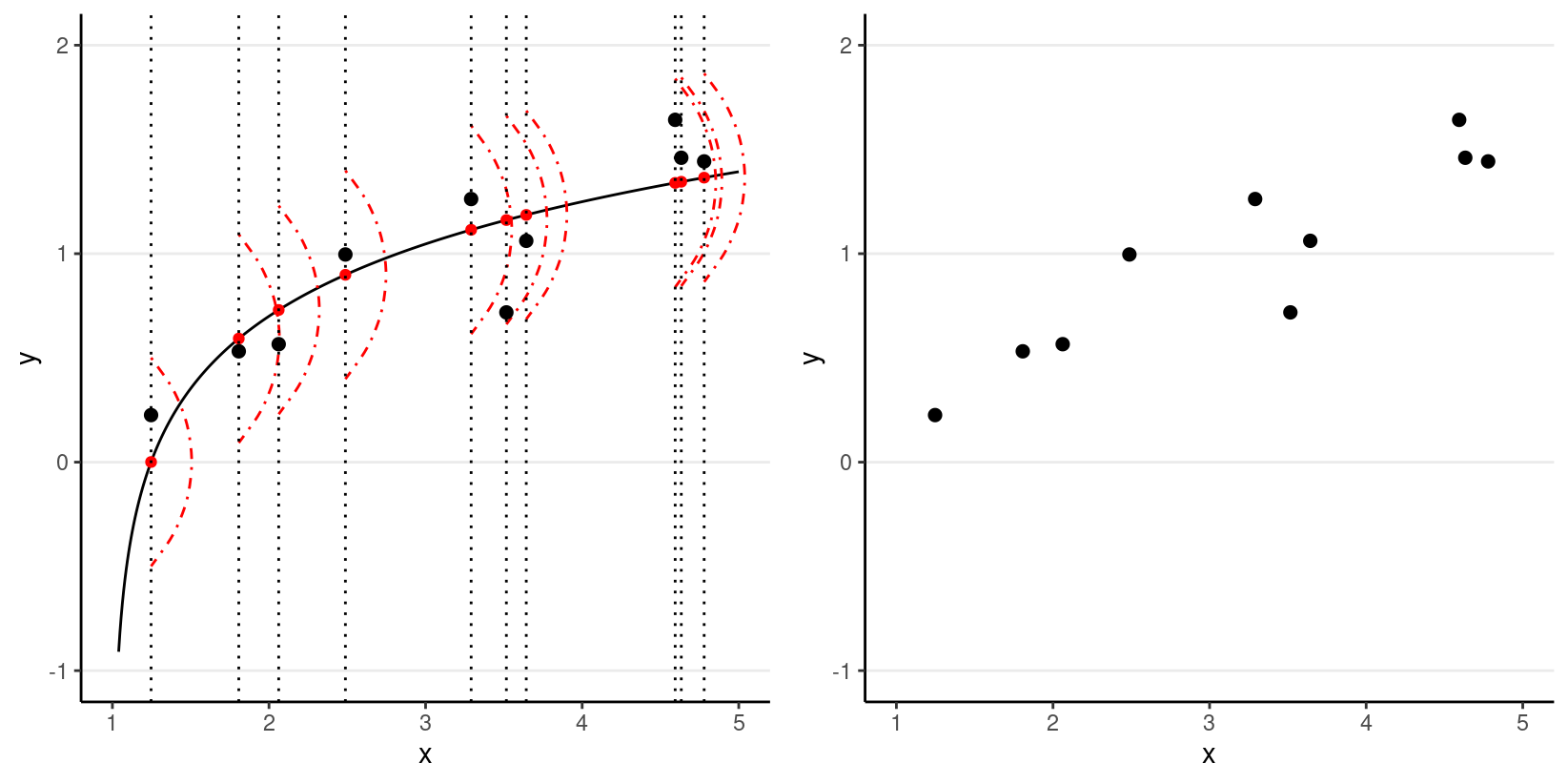
We can write the systematic part as:
E(Y∣X)=f(X;β1,β2,…),
where β1, β2, … are the parameters for some arbitrary function f(⋅). The random part is about P(Y∣X), which can take some arbitrary form of distributions. In reality, even if such a model holds, we do not know what f(⋅) and the true distribution of Y∣X are, as we only have data like those illustrated in the graph on the right above.
Overview of GLM
Under the GLM framework, with one predictor, we have models in the form
Yi∼Dist(μi,τ)g(μi)=ηiηi=β0+β1Xi
A GLM model has three components:
- Conditional distribution of Y
(e.g., Dist = Normal, Poisson, Binomial, Bernoulli)
- Linear predictor η, which is a linear combination of the predictor
- Link function g(⋅), which maps μ to η
In a GLM, one selects distributions like Dist = Normal, Poisson, Binomial, Bernoulli, etc. The distribution has a mean parameter μi and may have a dispersion parameter, τ. An intermediate step in GLM is transforming μi to ηi. ηi is called the linear predictor, which is the linear function of the predictors. In linear models, we directly model the conditional mean, μi, as the same as ηi. However, to allow for the possibility of μi being a nonlinear function of the predictors, in GLM we transform μi by applying a link function, g(⋅), so that, even though we ηi to be linear in the coefficients, μi=g−1(ηi) will be a nonlinear function of the coefficients as long as the link function is not linear. This step is needed to ensure that the predicted values will not be out of range.
The table below includes some commonly used GLMs
| outcome type | Support | Distributions | Link |
|---|---|---|---|
| continuous | [−∞, ∞] | Normal | Identity |
| count (fixed duration) | {0, 1, …} | Poisson | Log |
| count (known # of trials) | {0, 1, …, N} | Binomial | Logit |
| binary | {0, 1} | Bernoulli | Logit |
| ordinal | {0, 1, …, K} | categorical | Logit |
| nominal | K-vector of {0, 1} | categorical | Logit |
| multinomial | K-vector of {0, 1, …, K} | categorical | Logit |
Linear Regression
The linear regression model assumes that
- the function for the systematic component, f(⋅), is a linear function (in the βs),
- Y∣X is normally distributed, and
- Yi’s are conditionally exchangeable given X with equal variance σ2 (which can be relaxed).
Under these conditions, we have a model
Yi∼N(μi,σ)μi=ηiηi=β0+β1Xi
With parameters: - β0: regression intercept; predicted Y value for observations with X = 0 - β1: regression slope/coefficient; predicted difference in Y for one unit difference in X - σ: standard deviation of prediction error; roughly speaking, the margin of error in prediction
Example
We will use an example based on the “bread and peace” model by political scientist Douglas Hibbs, which can be used to forecast the U.S. presidential election outcome based on some weighted metric of personal income growth. The example is taken from chapter 7 of the book Regression and Other Stories.
Below is a graph showing the data from 1952 to 2012, with one variable being personal income growth in the four years prior to an election, and the other being the vote share (in %) of the incumbent’s party.
# Economy and elections data
hibbs <- read.table(
"https://github.com/avehtari/ROS-Examples/raw/master/ElectionsEconomy/data/hibbs.dat",
header = TRUE
)
ggplot(hibbs, aes(x = growth, y = vote, label = year)) +
geom_point() +
ggrepel::geom_text_repel() +
labs(x = "Average recent growth in personal income",
y = "Incumbent party's vote share (%)")
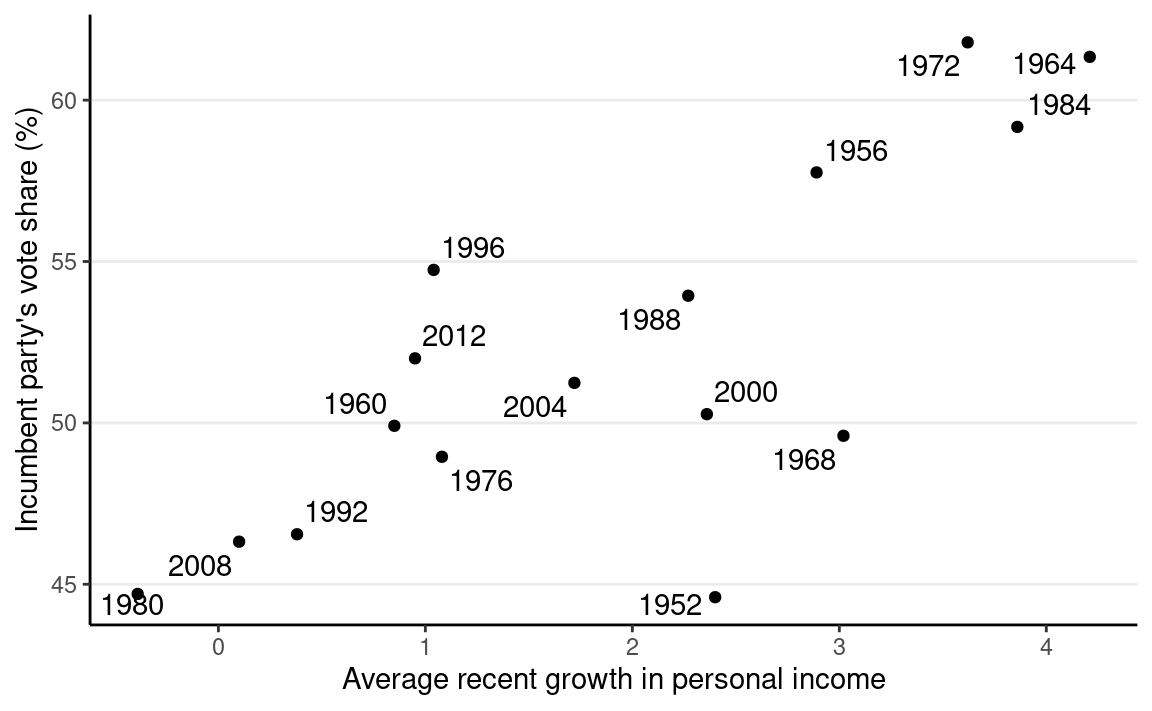
Model and Priors
Model:
votei∼N(μi,σ)μi=β0+β1growthi
σ: SD (margin) of prediction error
Priors:
β0∼N(45,10)β1∼N(0,10)σ∼t+4(0,5)
With Stan
Once you’ve written the model, it’s straightforward to code the model in Stan to perform MCMC sampling, like the code below.
data {
int<lower=0> N; // number of observations
vector[N] y; // outcome;
vector[N] x; // predictor;
}
parameters {
real beta0; // regression intercept
real beta1; // regression coefficient
real<lower=0> sigma; // SD of prediction error
}
model {
// model
y ~ normal(beta0 + beta1 * x, sigma);
// prior
beta0 ~ normal(45, 10);
beta1 ~ normal(0, 10);
sigma ~ student_t(4, 0, 5);
}
generated quantities {
vector[N] y_rep; // place holder
for (n in 1:N)
y_rep[n] = normal_rng(beta0 + beta1 * x[n], sigma);
}m1 <- stan(
here::here("stan", "linear_reg.stan"),
data = list(N = nrow(hibbs),
y = hibbs$vote,
x = hibbs$growth)
)
With brms
While Stan is very flexible, because GLM is so widely used, some
authors have created packages that would further simplify the fitting of
Bayesian GLMs (and some other models). One of those packages is
brms, which I believe stands for “Bayesian regression
models with Stan.” It allows one to do MCMC sampling using Stan, but
with syntax similar to that in R functions lm(),
glm(), and lme4::lmer(). brms
probably supports more models than any R packages that statisticians
routinely used; there are models, like factor analysis, that are not
directly supported. If you came across some fancy regression models,
chances are you can do something similar in brms. You can
find some resources for learning brms on this page: https://paul-buerkner.github.io/brms/
Set priors
brms comes with some default prior options, but I
recommend you always check what priors are used and think about whether
they make sense for your data. You can use get_priors() to
show the default priors used in brms.
#> prior class coef group resp dpar nlpar lb ub
#> (flat) b
#> (flat) b growth
#> student_t(3, 50.8, 6.1) Intercept
#> student_t(3, 0, 6.1) sigma 0
#> source
#> default
#> (vectorized)
#> default
#> defaultTo do MCMC sampling, we use the brm() function. The
first argument is a formula in R. The variable before ~ is
the outcome, whereas the ones after ~ are the predictors.
For example,
vote ~ 1 + growthmeans the model
E(votei)=β0(1)+β1(growthi).
Usually, I write vote ~ 1 + growth as
vote ~ growth, as the 1 + part is
automatically added.
m1_brm <- brm(
# Y (vote) = beta0 + beta1 (growth)
vote ~ growth,
data = hibbs,
# Normal distribution with identity link
family = gaussian(link = "identity"),
# Overwrite the default priors
prior = c(
# prior for beta0
prior(normal(45, 10), class = "Intercept"),
# prior for beta1 (and beta2, ..., if > 1 predictors)
prior(normal(0, 10), class = "b"),
# prior for sigma
prior(student_t(4, 0, 5), class = "sigma")
),
sample_prior = TRUE, # also sample the prior distributions
iter = 4000, # default is 4 chains, 2000 iterations each
seed = 31143
)
#>
#> SAMPLING FOR MODEL '88895eae8bd1139e161f2e157dbb72d7' NOW (CHAIN 1).
#> Chain 1:
#> Chain 1: Gradient evaluation took 1.1e-05 seconds
#> Chain 1: 1000 transitions using 10 leapfrog steps per transition would take 0.11 seconds.
#> Chain 1: Adjust your expectations accordingly!
#> Chain 1:
#> Chain 1:
#> Chain 1: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 1: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 1: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 1: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 1: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 1: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 1: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 1: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 1: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 1: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 1: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 1: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 1:
#> Chain 1: Elapsed Time: 0.04202 seconds (Warm-up)
#> Chain 1: 0.039389 seconds (Sampling)
#> Chain 1: 0.081409 seconds (Total)
#> Chain 1:
#>
#> SAMPLING FOR MODEL '88895eae8bd1139e161f2e157dbb72d7' NOW (CHAIN 2).
#> Chain 2:
#> Chain 2: Gradient evaluation took 8e-06 seconds
#> Chain 2: 1000 transitions using 10 leapfrog steps per transition would take 0.08 seconds.
#> Chain 2: Adjust your expectations accordingly!
#> Chain 2:
#> Chain 2:
#> Chain 2: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 2: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 2: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 2: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 2: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 2: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 2: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 2: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 2: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 2: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 2: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 2: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 2:
#> Chain 2: Elapsed Time: 0.041465 seconds (Warm-up)
#> Chain 2: 0.040759 seconds (Sampling)
#> Chain 2: 0.082224 seconds (Total)
#> Chain 2:
#>
#> SAMPLING FOR MODEL '88895eae8bd1139e161f2e157dbb72d7' NOW (CHAIN 3).
#> Chain 3:
#> Chain 3: Gradient evaluation took 6e-06 seconds
#> Chain 3: 1000 transitions using 10 leapfrog steps per transition would take 0.06 seconds.
#> Chain 3: Adjust your expectations accordingly!
#> Chain 3:
#> Chain 3:
#> Chain 3: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 3: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 3: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 3: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 3: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 3: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 3: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 3: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 3: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 3: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 3: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 3: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 3:
#> Chain 3: Elapsed Time: 0.040855 seconds (Warm-up)
#> Chain 3: 0.037823 seconds (Sampling)
#> Chain 3: 0.078678 seconds (Total)
#> Chain 3:
#>
#> SAMPLING FOR MODEL '88895eae8bd1139e161f2e157dbb72d7' NOW (CHAIN 4).
#> Chain 4:
#> Chain 4: Gradient evaluation took 5e-06 seconds
#> Chain 4: 1000 transitions using 10 leapfrog steps per transition would take 0.05 seconds.
#> Chain 4: Adjust your expectations accordingly!
#> Chain 4:
#> Chain 4:
#> Chain 4: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 4: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 4: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 4: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 4: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 4: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 4: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 4: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 4: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 4: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 4: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 4: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 4:
#> Chain 4: Elapsed Time: 0.040977 seconds (Warm-up)
#> Chain 4: 0.040616 seconds (Sampling)
#> Chain 4: 0.081593 seconds (Total)
#> Chain 4:You can print the results
print(m1_brm, digits = 2) # `digits = 2` is default
#> Family: gaussian
#> Links: mu = identity; sigma = identity
#> Formula: vote ~ growth
#> Data: hibbs (Number of observations: 16)
#> Draws: 4 chains, each with iter = 4000; warmup = 2000; thin = 1;
#> total post-warmup draws = 8000
#>
#> Population-Level Effects:
#> Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
#> Intercept 46.22 1.75 42.73 49.66 1.00 5961 4575
#> growth 3.04 0.75 1.54 4.47 1.00 5775 4413
#>
#> Family Specific Parameters:
#> Estimate Est.Error l-95% CI u-95% CI Rhat Bulk_ESS Tail_ESS
#> sigma 4.01 0.80 2.81 5.88 1.00 5147 5039
#>
#> Draws were sampled using sampling(NUTS). For each parameter, Bulk_ESS
#> and Tail_ESS are effective sample size measures, and Rhat is the potential
#> scale reduction factor on split chains (at convergence, Rhat = 1).The coefficients under Population-Level Effects are the
βs, and those under
Family Specific Parameters are the τs (scale parameters). The one labeled
Intercept is β0,
and the one labeled growth is the coefficient for the
growth predictor, which is β1.
Here’s a paragraph for the results:
The model predicts that when personal income growth is 0, the vote share for the incumbent party is 46.22%, 90% CI [43.36%, 49.07%]. A 1 unit difference in personal income growth corresponds to a difference in vote share by 3.04 precentage points, 90% CI [1.81, 4.23].
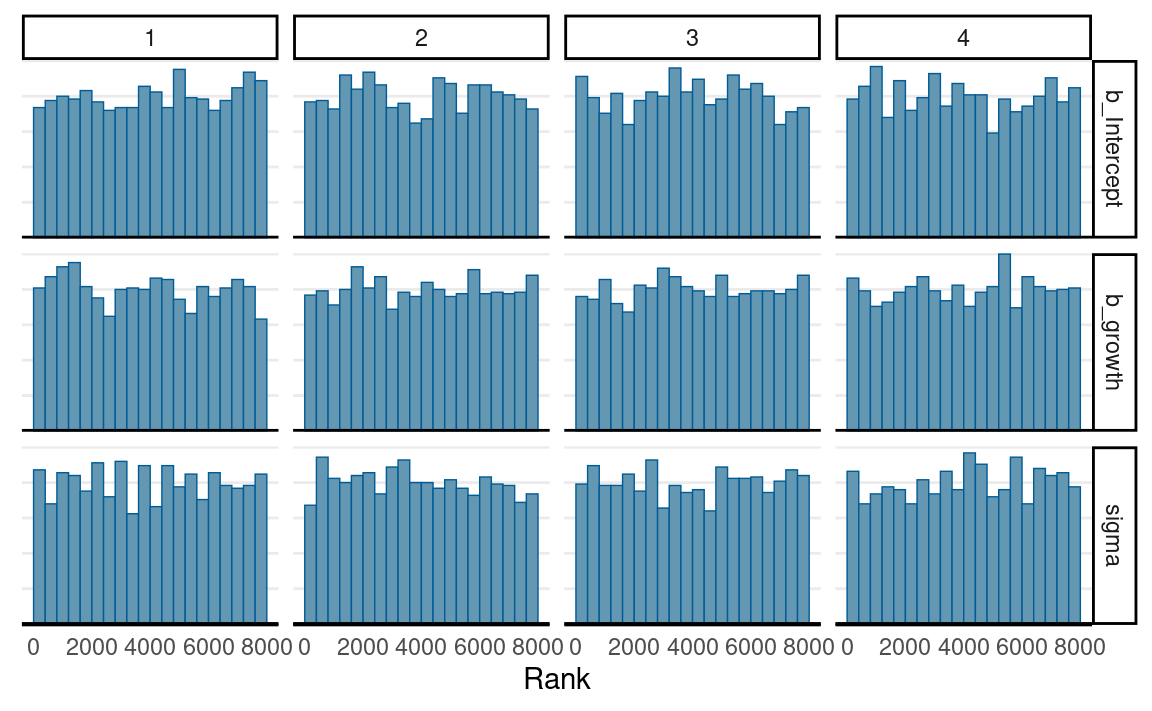
Convergence
mcmc_trace(m1_brm,
pars = c("b_Intercept", "b_growth", "sigma"))

mcmc_rank_hist(m1_brm,
pars = c("b_Intercept", "b_growth", "sigma"))
Posterior plots
There are many ways to visualize the results of a regression model. The most important thing is to be as familiar with what the results mean as possible. Statistics is not some magic that gives you some numbers that either support or do not support your theory or hypothesis. It is a way to describe your data. If you do not want to do the work to understand what your data tell you, why bother to collect the data in the first place?
Posterior density
Posterior distributions of the three parameters

You can also combine the density plot and the trace plot
plot(m1_brm)

Plot regression prediction
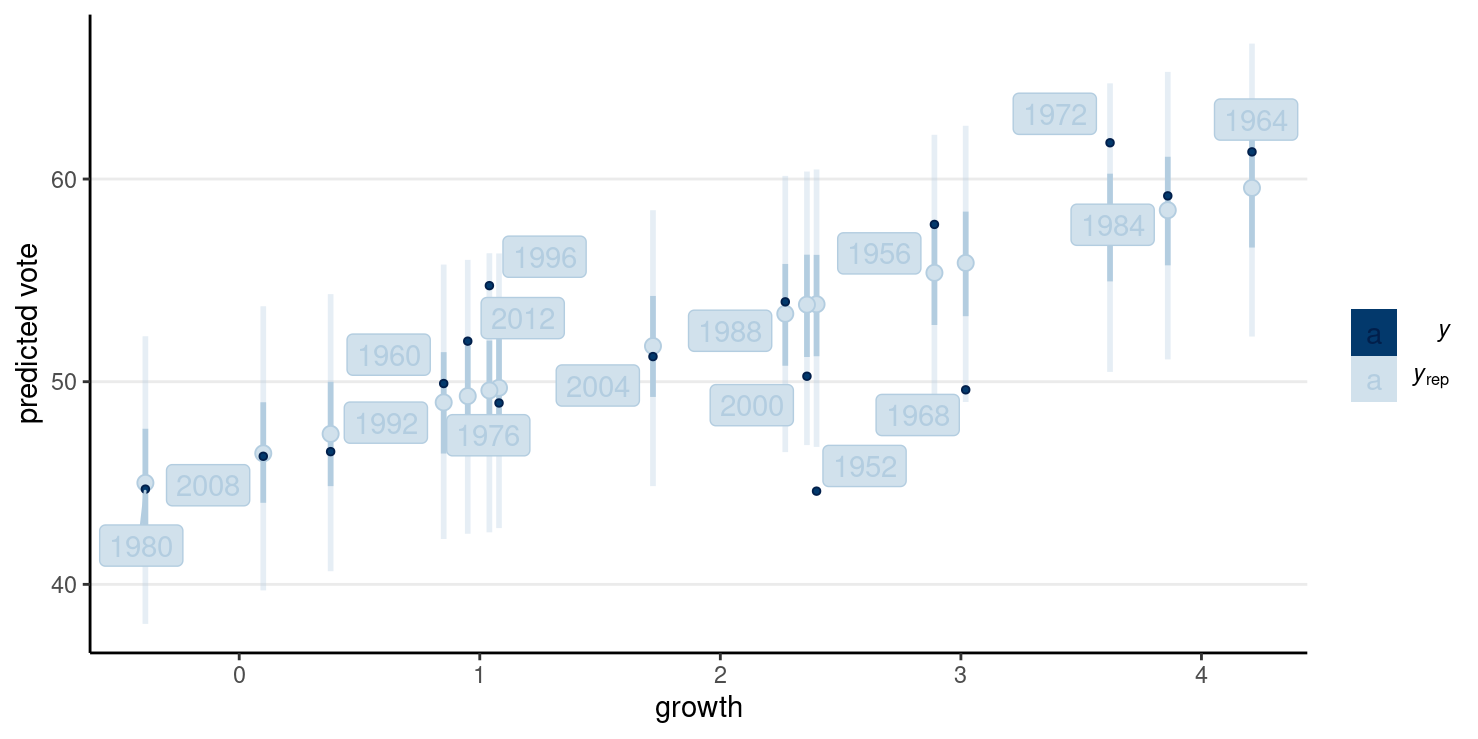
The plot below shows the 50% and 90% prediction intervals based on the posterior samples and the model. For example, with the 90% intervals, one expects 90% of the data should be within those intervals. If you see many data points lying outside the intervals, the linear model is not a good fit.
pp_check(
m1_brm,
type = "intervals",
x = "growth"
) +
labs(x = "growth", y = "predicted vote") +
ggrepel::geom_label_repel(
aes(y = hibbs$vote, label = hibbs$year)
)

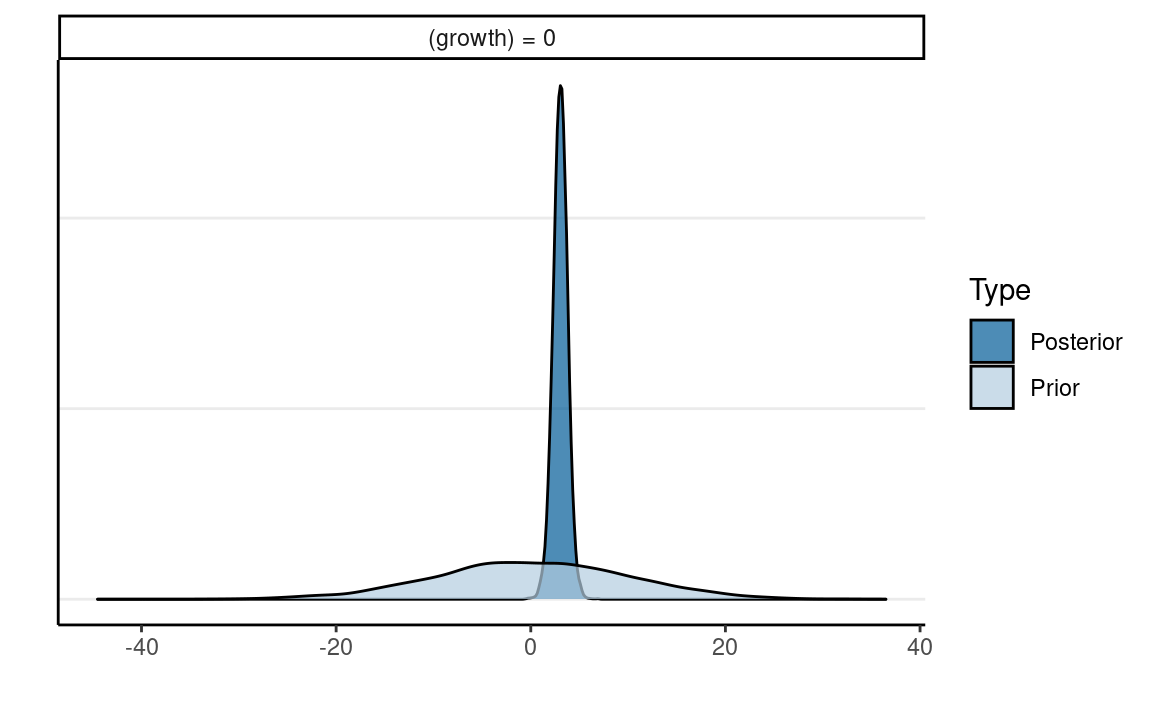
Plot priors and posteriors together
h_growth <- hypothesis(m1_brm,
hypothesis = c("growth = 0")
)
plot(h_growth)
Table
You can use the modelsummary::msummary() function to get
a nicely formatted table of GLM coefficients. We’ll talk about things
like ELPD, LOOIC, and WAIC later in the course.
msummary(m1_brm, statistic = "conf.int", fmt = 2)
| Model 1 | |
|---|---|
| b_Intercept | 46.22 |
| [42.62, 49.53] | |
| b_growth | 3.04 |
| [1.54, 4.47] | |
| sigma | 3.91 |
| [2.72, 5.67] | |
| Num.Obs. | 16 |
| ELPD | −46.1 |
| ELPD s.e. | 3.6 |
| LOOIC | 92.3 |
| LOOIC s.e. | 7.2 |
| WAIC | 92.0 |
| RMSE | 3.52 |
Predicting a New Data Point
In the four years before 2016, the weighted average personal income growth was 2.0 (based on Hibbs’ calculation). So based on the model, we can obtain a posterior distribution for the predicted vote share of the Democratic Party, which is the incumbent party prior to the 2016 presidential election.
pp_growth_eq_2 <- posterior_predict(m1_brm,
newdata = list(growth = 2)
)
colnames(pp_growth_eq_2) <- "y_tilde"
mcmc_dens(pp_growth_eq_2)
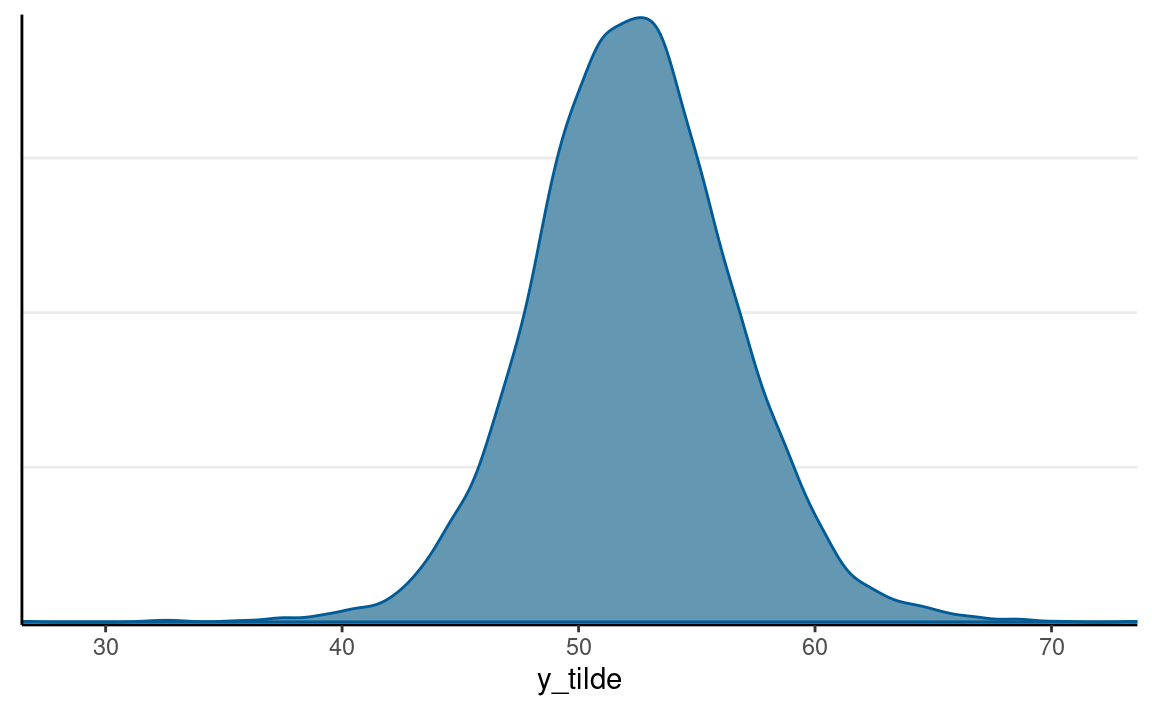
The probability of incumbent’s vote share > 50% with average income growth = 2 is 0.712375. The actual outcome of the election was that the Democratic Party received about 51.1% of the votes among the two parties, so it was below the model prediction but within the range of possible outcomes. Of course, we know that the actual election outcome is based on the electoral college, not the majority vote.
Robust Regression
m1_robust <- brm(vote ~ growth,
data = hibbs,
family = student(link = "identity"),
prior = c(
prior(normal(45, 10), class = "Intercept"),
prior(normal(0, 10), class = "b"),
prior(student_t(4, 0, 5), class = "sigma"),
prior(gamma(2, 0.1), class = "nu")
),
sample_prior = TRUE,
iter = 4000,
seed = 31143
)
#>
#> SAMPLING FOR MODEL '99a04619a1f72e8dcd46dfc45e6d584b' NOW (CHAIN 1).
#> Chain 1:
#> Chain 1: Gradient evaluation took 1.3e-05 seconds
#> Chain 1: 1000 transitions using 10 leapfrog steps per transition would take 0.13 seconds.
#> Chain 1: Adjust your expectations accordingly!
#> Chain 1:
#> Chain 1:
#> Chain 1: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 1: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 1: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 1: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 1: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 1: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 1: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 1: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 1: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 1: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 1: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 1: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 1:
#> Chain 1: Elapsed Time: 0.080852 seconds (Warm-up)
#> Chain 1: 0.080073 seconds (Sampling)
#> Chain 1: 0.160925 seconds (Total)
#> Chain 1:
#>
#> SAMPLING FOR MODEL '99a04619a1f72e8dcd46dfc45e6d584b' NOW (CHAIN 2).
#> Chain 2:
#> Chain 2: Gradient evaluation took 1.4e-05 seconds
#> Chain 2: 1000 transitions using 10 leapfrog steps per transition would take 0.14 seconds.
#> Chain 2: Adjust your expectations accordingly!
#> Chain 2:
#> Chain 2:
#> Chain 2: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 2: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 2: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 2: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 2: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 2: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 2: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 2: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 2: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 2: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 2: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 2: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 2:
#> Chain 2: Elapsed Time: 0.08474 seconds (Warm-up)
#> Chain 2: 0.0754 seconds (Sampling)
#> Chain 2: 0.16014 seconds (Total)
#> Chain 2:
#>
#> SAMPLING FOR MODEL '99a04619a1f72e8dcd46dfc45e6d584b' NOW (CHAIN 3).
#> Chain 3:
#> Chain 3: Gradient evaluation took 1.3e-05 seconds
#> Chain 3: 1000 transitions using 10 leapfrog steps per transition would take 0.13 seconds.
#> Chain 3: Adjust your expectations accordingly!
#> Chain 3:
#> Chain 3:
#> Chain 3: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 3: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 3: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 3: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 3: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 3: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 3: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 3: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 3: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 3: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 3: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 3: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 3:
#> Chain 3: Elapsed Time: 0.07375 seconds (Warm-up)
#> Chain 3: 0.076318 seconds (Sampling)
#> Chain 3: 0.150068 seconds (Total)
#> Chain 3:
#>
#> SAMPLING FOR MODEL '99a04619a1f72e8dcd46dfc45e6d584b' NOW (CHAIN 4).
#> Chain 4:
#> Chain 4: Gradient evaluation took 1.2e-05 seconds
#> Chain 4: 1000 transitions using 10 leapfrog steps per transition would take 0.12 seconds.
#> Chain 4: Adjust your expectations accordingly!
#> Chain 4:
#> Chain 4:
#> Chain 4: Iteration: 1 / 4000 [ 0%] (Warmup)
#> Chain 4: Iteration: 400 / 4000 [ 10%] (Warmup)
#> Chain 4: Iteration: 800 / 4000 [ 20%] (Warmup)
#> Chain 4: Iteration: 1200 / 4000 [ 30%] (Warmup)
#> Chain 4: Iteration: 1600 / 4000 [ 40%] (Warmup)
#> Chain 4: Iteration: 2000 / 4000 [ 50%] (Warmup)
#> Chain 4: Iteration: 2001 / 4000 [ 50%] (Sampling)
#> Chain 4: Iteration: 2400 / 4000 [ 60%] (Sampling)
#> Chain 4: Iteration: 2800 / 4000 [ 70%] (Sampling)
#> Chain 4: Iteration: 3200 / 4000 [ 80%] (Sampling)
#> Chain 4: Iteration: 3600 / 4000 [ 90%] (Sampling)
#> Chain 4: Iteration: 4000 / 4000 [100%] (Sampling)
#> Chain 4:
#> Chain 4: Elapsed Time: 0.082871 seconds (Warm-up)
#> Chain 4: 0.083182 seconds (Sampling)
#> Chain 4: 0.166053 seconds (Total)
#> Chain 4:pp_check(
m1_robust,
type = "intervals",
x = "growth"
) +
labs(x = "growth", y = "predicted vote") +
ggrepel::geom_label_repel(
aes(y = hibbs$vote, label = hibbs$year)
)
Poisson Regression
The Poisson GLM is used to model count outcomes. Count outcomes are non-negative discrete integers. Remember, with GLM, we are modeling the mean of the outcome, μ. Therefore, we need to make sure μ is non-negative, so we need a link function that can map η from the whole real line to non-negative numbers; by far, the most commonly used link function is the logarithmic transformation, g(μ)=log(μ).
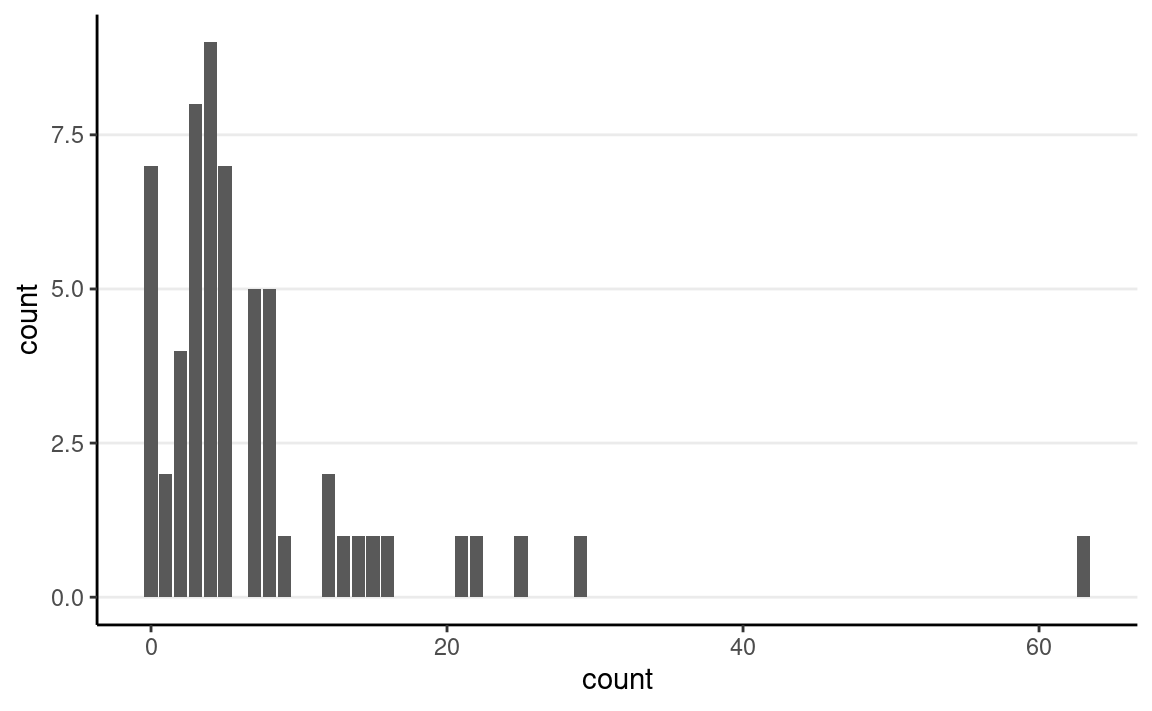
Here’s an example data set recording effect of anticonvulsant therapy in epilepsy. The outcome variable is the number of seizures in the two-week window prior to the last of the four visits.
First check the distribution of the counts:
epilepsy4 <- dplyr::filter(epilepsy, visit == 4)
epilepsy4$Trt <- factor(epilepsy4$Trt)
ggplot(epilepsy4, aes(x = count)) +
geom_bar()
And the box plots
set.seed(1417)
ggplot(epilepsy4, aes(x = Trt, y = count)) +
geom_boxplot() +
geom_jitter(width = 0.05)

Model and Priors
Model:
counti∼Pois(μi)log(μi)=ηiηi=β0+β1Trti
Priors:
β0∼N(3,2.5)β1∼N(0,1)
Predicted seizure rate = exp(β0+β1)=exp(β0)exp(β1) for Trt = 1; exp(β0) for Trt = 0
β1 = mean difference in log rate of seizure; exp(β1) = ratio in rate of seizure
MCMC Sampling With
brms
m2 <- brm(count ~ Trt,
data = epilepsy4,
family = poisson(link = "log"),
prior = c(
prior(normal(1, 3), class = "Intercept"),
prior(normal(0, 1), class = "b")
),
iter = 4000,
seed = 31143
)
Interpretations
Because of the nonlinear link function, one needs to be careful in interpreting the coefficients. With the log link, it is common to obtain the exponentiated coefficient. With the exponentiated coefficient, for every unit difference in X, the predicted rate of seizure occurrence is multiplied by exp(β1) times. Here is the usual step for obtaining the posterior distributions of the transformed parameters:
m2_summary <- as_draws_array(m2) %>%
mutate_variables(exp_beta0 = exp(b_Intercept),
exp_beta1 = exp(b_Trt1)) %>%
summarize_draws()
So here is a paragraph for the example:
The model predicts that the mean seizure rate in two weeks for the control condition is -334.41, 90% CI [-336.33, -333.48]; the exponentiated coefficient for the treatment indicator is 7.96, 90% CI [7.12, 8.84], meaning that on average, the treatment reduces seizure rate by -784.04% to -611.84%.
Rootogram
There is also a useful graphical tool, the rootogram, for diagnosing count models.
pp_check(m2, type = "rootogram", style = "hanging")
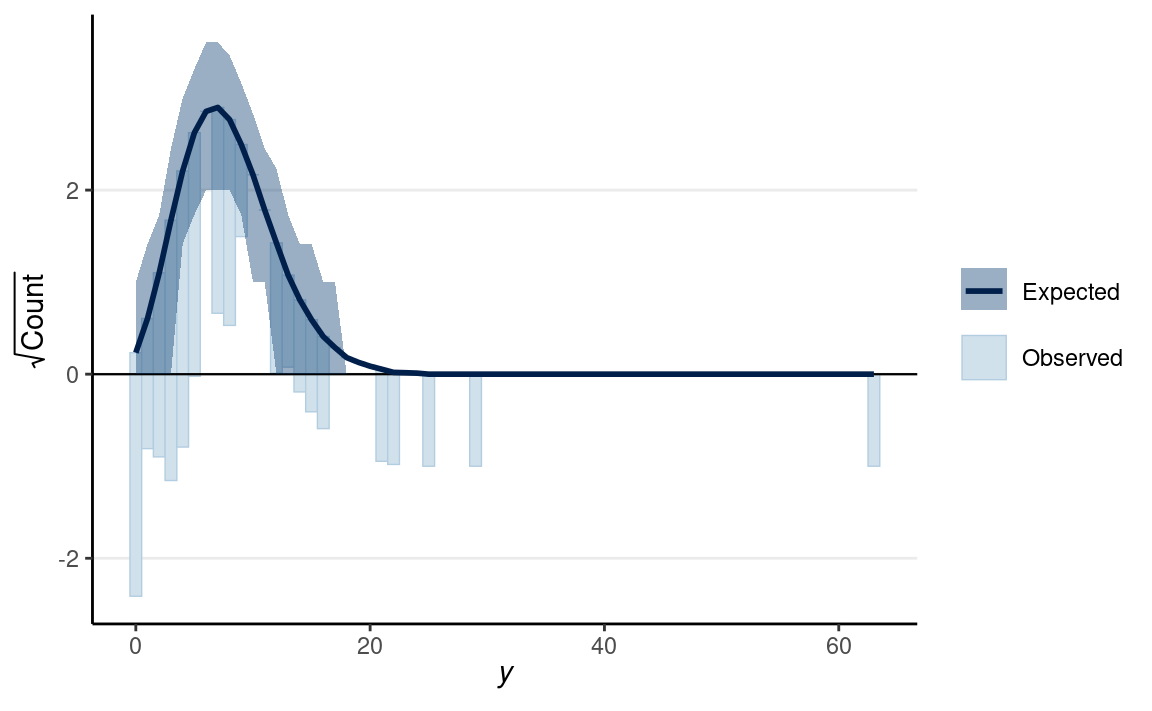
If the fit is good, the bars should be close to touching the horizontal x-axis. Thus, the fit was not good in the above figure. Models that accommodate overdispersion and excessive zeros may be needed.
Negative Binomial Model
The negative binomial distribution is usually used to handle overdispersion in count data. The idea is that even with the same predictor value, each individual has a different rate of occurrence for an event, which is highly plausible for the epilepsy data. This class will not get into the details of the negative binomial, but you can check out this paper or this book.
m2_nb <- brm(count ~ Trt,
data = epilepsy4,
family = negbinomial(link = "log"),
prior = c(
prior(normal(1, 3), class = "Intercept"),
prior(normal(0, 1), class = "b")
),
iter = 4000,
seed = 31143
)
pp_check(m2_nb, type = "rootogram", style = "hanging")
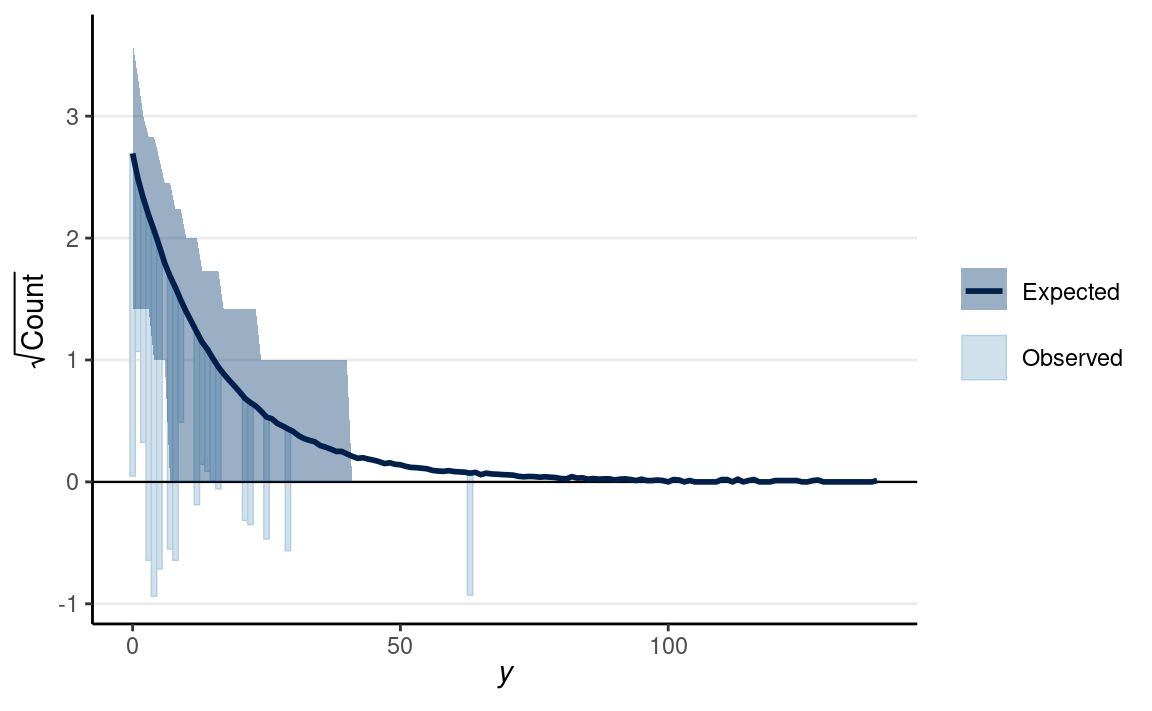
We’ll continue with some other models in GLM later in the class. But we’ll discuss regression with multiple predictors and causal thinking first.